The CEA-Grenoble Nano-Characterization Platform (PFNC) and the LEMMA group in particular, are dedicated to improving cutting-edge electron microscopy techniques in support to materials science. However, several of the techniques we are developing are also of significant interest in the life sciences. This is the case for FIB-SEM nano-tomography, EDX or EELS spectroscopy, and TOF-SIMS imaging. All of these initially “material sciences” techniques are still little used by biologists due to the scarcity of advanced instruments and available expertise. Through collaborations with different groups of biologists,
our aim is to disseminate these emerging methods and expand their use to tackle biological problems, in particular in correlative workflows.
Most structural analyses of cells and tissues still result from two-dimensional images made by optical or electron microscopy. Although of great interest, this give only limited, and sometimes misleading, information about the inherently three-dimensional nature of these objects. In this respect, focused ion beam scanning electron microscopy
(FIB-SEM) constitutes a revolution for cell imaging in biology. It allows the 3D visualization of small structures (from cells to tissues and organisms) by successive SEM imaging of a surface abraded slice by slice using gallium ions. This makes it possible to obtain images with an isotropic spatial resolution of a few nanometres, close to that of transmission electron microscopy, but with the immense advantage of not having a simple projection of the object, but its entire volume in 3D. Subsequent segmentations of the image stack allows reconstruction of complete 3D models of tissues and organelles, and extraction of quantitative data at the nanoscale.
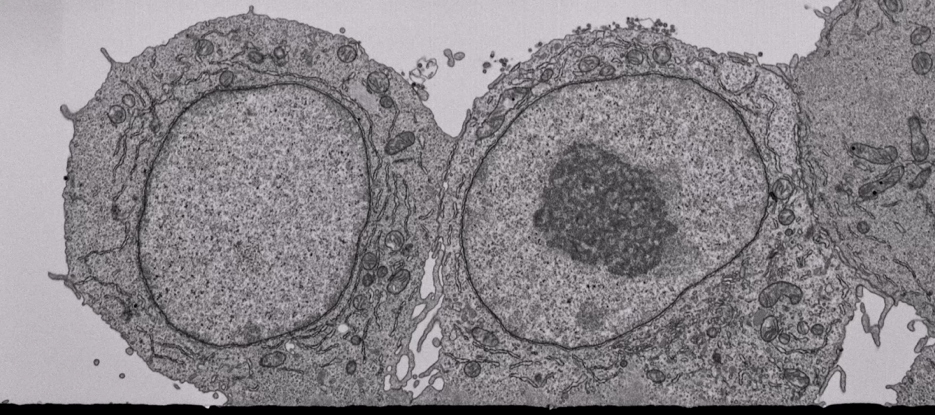
Murine macrophages on a glass substrate.
2683 images of 6824 x 3053 pixels with a 4 nm isotropic voxel size – 71 h acquisition.
BSE images @ 1.5 kV – Volume: 27.3 x 12.2 x 10.7 µm
LEMMA has a long experience in
developing FIB-SEM as a tool for subcellular imaging, especially in conjunction with other imaging modalities or in cryo mode. We are also working on the development of complementary techniques (low voltage STEM & SEM imaging, array tomography), as well as on the
segmentation of the 3D images to extract 3D quantitative data. We have also a collaboration with the electron microscopy platform of the IBS (Benoît Gallet, Guy Schoehn group) in particular for all the crucial aspects of sample preparation and cryopreparation.

 Recent collaborations and results
Recent collaborations and results
We use volume electron microscopy in collaborative projects to explore a variety of cellular processes. Among the various themes studied recently, we can cite
[1-7]:
- The structure and function of the photosynthetic machinery in marine organisms
(with the group of Giovanni Finazzi, LPCV) - Photosymbiosis interactions between unicellular eukaryotes and plankton
(with the group of Johan Decelle, LPCV)
- The structure of the liver and the fate of NP-Ag in hepatocyte spheroids
(with the groups of Aurélien Deniaud – LCBM)
- The structure of snow algae
(with the group of Eric Maréchal, LPCV) - The ultrastructural morphology of the astrocytes
(with the group of Karin Pernet Gallet, GIN)
3D reconstructions with their subcellular structures of a diatom (Phaeodactylum tricornutum) (top) and of a coccolithophore (Emiliania huxleyi) (bottom).
 Chemical imaging with EDX, EELS or TOF-SIMS spectroscopy
Chemical imaging with EDX, EELS or TOF-SIMS spectroscopy Another LEMMA area of expertise is the chemical imaging of samples at the nanometric scale. We use several techniques for this purpose.
Energy dispersive X-ray spectroscopy (EDS) and electron energy loss spectroscopy (EELS) allow the elemental imaging at the nanoscale on TEM-prepared samples. Although known from a long time, remarkable technical progress has recently taken place with these techniques, especially in STEM mode, and they are still underused for biological studies given their possibilities. For example, there are very well suited to follow the fate of xenobiotic nanoparticles in tissues or organisms
[8-12].
Time-of-Flight Secondary Ions Mass Spectrometry (TOF-SIMS) is another widely used technique in materials science with great potential for the life sciences. A pulsed beam of primary ions removes molecules from the outermost surface of a sample, these secondary particles are accelerated, and their mass is determined by measuring the time at which they reach the detector. Ions and isotopes, representatives of molecular compounds, can be detected with a mass range of 0-10,000 amu, a sensitivity in the ppm range, and a high spatial resolution (< 100 nm), with a full mass spectrum recorded for every pixel. This allows to produce images for any compound of interest, and to investigate regions of interest for their chemical composition. TOF-SIMS make it possible to visualize the local distribution of metabolites such as lipids at the subcellular scale and therefore allow decisive progress in the understanding of the associated biological mechanisms. This is a tool of choice for localizing lipids, sugars or nutrients at the subcellular level.
At PFNC (and in collaboration with LETI), we aim to further develop this technique and expand its use in solving biological problems. We are particularly interested in the development of
cryogenic methodologies to observe samples as close as possible to their native state, and in
3D correlative approaches with volume electron microscopy to mix local chemical information with structural information.

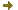 Main equipment available for biological studies at PFNC
For FIB-SEM and volume-SEM imagin
Main equipment available for biological studies at PFNC
For FIB-SEM and volume-SEM imaging

Zeiss CrossBeam 550 microscope equipped with a Quorum cryo stage and Atlas 5 software, entirely dedicated to 3D acquisitions

ThermoFisher Helios 5 Plasma FIB (with Xe ions)

Zeiss Merlin SEM with detector for SEM & STEM imaging and EDX spectroscopy

Workstations for image processing and data analysis (with Fiji/ImageJ, Ilastik, 3DSlicer, Dragonfly, Avizo, Reactiv’IP IPSDK…)
For Chemical imaging and spectroscopy

ThermoFisher (S)TEM microscopes (Titan Ultimate, Titan Themis, Osiris) with various EDS & EELS detectors (Brucker & Gatan)

TOF-SIMS (PHI NanoTOF & IONTOF)


 Contact and collaborations
Contact and collaborations
We are always open to new collaborations (within the time available on the instruments): if you are interested in exploring the possibility of working with us, contact us directly.
Contact:
Pierre-Henri Jouneau
 Recent publications
[1]
Recent publications
[1] Maier, U. G.
et al. Cell Biology of Organelles. in
The Molecular Life of Diatoms 265–286 (Springer, 2022).
[2] Cojocaru, R.
et al. A biological nanofoam: The wall of coniferous bisaccate pollen.
Science advances
8, eabd0892 (2022).
[3] Uwizeye, C.
et al. Morphological bases of phytoplankton energy management and physiological responses unveiled by 3D subcellular imaging.
Nature communications
12, 1–12 (2021).
[4] Sharma, V. R.
et al. Canalicular domain structure and function in matrix-free hepatic spheroids.
Biomaterials science
8, 485–496 (2020).
[5] Lupette, J.
et al. The architecture of lipid droplets in the diatom
Phaeodactylum tricornutum.
Algal Research
38, 101415 (2019).
[6] Decelle, J.
et al. Algal remodeling in a ubiquitous planktonic photosymbiosis.
Current Biology
29, 968–978 (2019).
[7] Flori, S.
et al. Plastid thylakoid architecture optimizes photosynthesis in diatoms.
Nature communications
8, 1–9 (2017).
[8] Suarez, V. T.
et al. Correlative transmission electron microscopy and high-resolution hard X-ray fluorescence microscopy of cell sections to measure trace element concentrations at the organelle level.
Journal of Structural Biology
213, 107766 (2021).
[9] Dussert, F.
et al. Evaluation of the dermal toxicity of InZnP quantum dots before and after accelerated weathering: Toward a safer-by-design strategy.
Frontiers in toxicology
3, 636976 (2021).
[10] Bobyk, L.
et al. Toxicity and chemical transformation of silver nanoparticles in A549 lung cells: Dose-rate-dependent genotoxic impact.
Environmental Science: Nano
8, 806–821 (2021).
[11] Marchioni, M.
et al. Safer-by-design biocides made of tri-thiol bridged silver nanoparticle assemblies.
Nanoscale Horizons
5, 507–513 (2020).
[12] Marchioni, M.
et al. A. Silver nanoparticle fate in mammals: Bridging
in vitro and
in vivo studies.
Coordination Chemistry Reviews
364, 118–136 (2018).