For instance, the formation of the so-called Solid Electrolyte Interface (SEI) is considered as one of the most important factors defining the overall battery behavior in terms of storage properties, charge transfer kinetics and irreversible capacity loss.
Li-ion batteries have a high energy density. The electronic transfers during charge/ discharge cycles are combined with Lithium exchange between electrodes through the electrolyte. Iron phosphates are used for positive electrodes and allows the Lithium to be intercalated or released.
Different synthesis batches of LiFePO4/C materials were prepared and their electrochemical properties as positive cathodes for lithium-ion batteries were evaluated. Using standard solid-state NMR conditions, such as a 7 mm magic-angle-spinning probe performing at low spinning rates, information on both intercalated and non-intercalated (stored on the grain boundaries) lithium was obtained. A sharp signal assigned to non-intercalated lithium could be observed by diluting the active material in silica. Correlations could be thus obtained between the amount of each type of Lithium and the electrochemical history and state of the material, revealing that the relative amount of surface lithium in a pristine LiFePO4/C material is rather constant and cannot be used as a criterion for its further specification. However, a drastic increase of this surface lithium was observed in the cathode materials of out-of-order batteries. As the cathode material recovered from the batteries after electrochemical testing was carefully washed before analysis, we can conclude that the non-intercalated lithium is strongly bound to the active material probably inside the so-called Solid Electrolyte Interface (SEI) layer at the surfaces of LiFePO4 particles. This work illustrates that solid-state Lithium NMR can allow rapid characterization and testing of LiFePO4/C cathode materials.
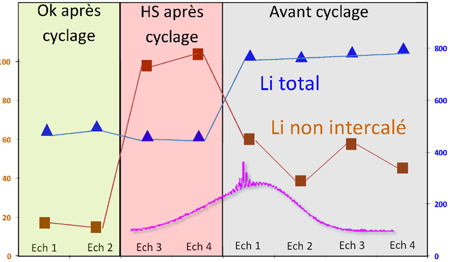
Amount of Li measured by NMR for 4 LiFePO4 samples, two of them were identified to give electrodes with good battery specifications, whereas the two others lead to deteriorated battery specifications. The figure shows the broad spectrum assigned to intercalated Li and the narrower spectrum of non-intercalated Li inside the SEI. The amounts of non-intercalated Li are higher for batteries with deteriorated specification.