Lithium-ion batteries play a central role in the development of numerous industrial applications such as electrified transport or the storage of intermittent renewable energies. Improving their performance, in particular their energy density and cycling stability, has become a major scientific and economic challenge. In the negative electrode (anode), lithium is alternately stored during charging, then released when the battery is discharged. At present, its standard material is graphite. One promising direction would be to replace it with silicon-based materials. Indeed, for an equivalent mass, silicon can store much more lithium than graphite, which translates into a better theoretical capacity for the battery (approximately 3600
vs. 372 mAh/g). Unfortunately, this is combined with major degradation problems linked to mechanical expansion (about 300% for pure Si) and the strong reactivity of Si leading to the formation of inert compounds, which will trap part of the lithium, and thus limit the cycling capacities.
Within the framework of the European project Sintbat carried out in collaboration with the CEA-Liten and various industrial partners (such as Varta),
teams from our laboratory and at the IRIG have been studying these silicon degradation mechanisms and ways to limit them. This research is based on the unique combination of structural characterisation techniques available at the Modeling and Exploration of Materials (MEM) laboratory and on large instruments (notably the ESRF). Thus, the combination of electron microscopy and electron energy loss spectroscopy (STEM-EELS) at the atomic scale, X-ray diffraction and NMR and XPS spectroscopy make it possible to apprehend phenomena at all relevant scales.
Researchers from our laboratory and SyMMES laboratory have recently been able to demonstrate the interest of using new nano-architectured materials composed of active amorphous silicon domains - and crystalline FeSi
2 nanoparticles dispersed in a graphite matrix. This hierarchical architecture improves long-term mechanical and cycling stability. The researchers propose an aging model at the nanometric scale (
Figure) to describe the evolution of the alloy during lithiation and thus explain the aging process after hundreds of cycles. During the volumetric variations due to lithiation/delithiation, the morphology of the a-Si /c-FeSi
2 alloy evolves from a core-shell structure (
Figure A) to a tree-branch structure (
Figure B) in which the continuous network of active a-Si remains intact, yielding capacity retention of nearly 70% after 700 cycles.
These results provide substantial knowledge on aging mechanisms which are essential to optimize the design of alloy-based active materials and electrode formulation.
This study involved
several teams at our laboratory and at IRIG: SGX, LEMMA and NRS teams from the Modeling and Exploration of Materials (MEM) laboratory, and the STEP team from the Molecular Systems and nanoMaterials for Energy and Health (
SyMMES) laboratory.
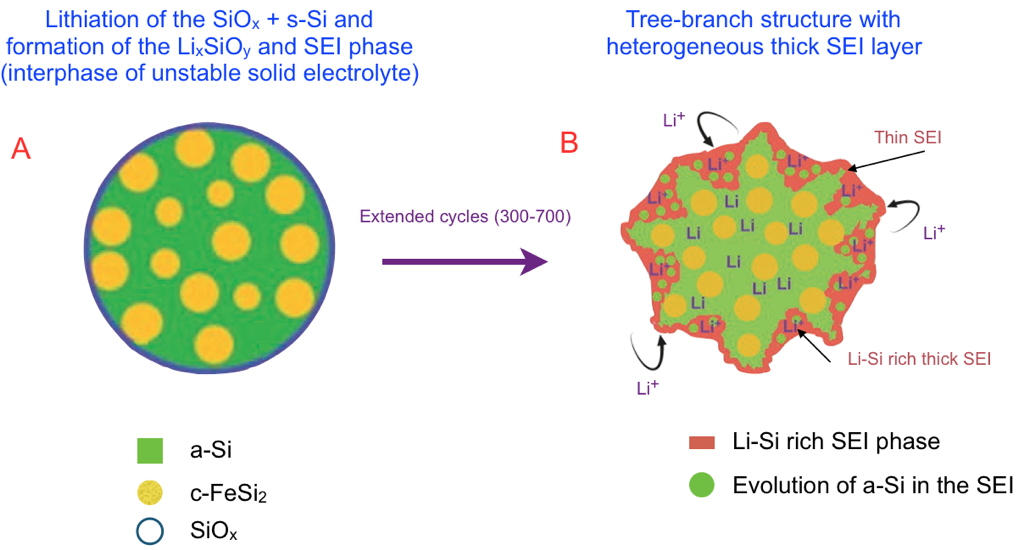
Schematic of the lithiation mechanism of the dual‐phase a‐Si/c‐FeSi
2 alloy particles over extended cycling. Different steps determine how the a‐Si/c‐FeSi
2 alloy evolves upon lithiation, only the first and last of which are represented. Successive lithiations lead to the formation of the new phases (Li
xSiO
y), and ultimately to the evolution towards a tree branch structure (after around 300–700 cycles) with an heterogeneous Li–Si rich thick solid electrolyte interphase (SEI). In this structure, the core is preserved, which explains the longevity of the batteries.